Paralytic Versus "Non-Paralytic" Polio: A Distinction without a Difference?
Richard L. Bruno, HD, PhD
American Journal of Physical Medicine and Rehabilitation, 2000; 79: 1-9.
Abstract
Non-paralytic polio (NPP) is commonly thought to be synonymous with "abortive polio," in which the poliovirus neither entered the Central Nervous System (CNS) nor damaged neurons. Described are two epidemic illness -- "The Summer Grippe" and Iceland disease -- apparently caused by a low virulence but neuropathic type 2 poliovirus, studies showing that neuronal lesions in the brain and spinal cord and muscle weakness were common in NPP, and epidemiologic studies documenting late-onset weakness and fatigue in 14% to 42% of NPP survivors. Clinicians should not require a history of paralytic polio, EMG evidence of denervation and new muscle weakness for a diagnosis of "Post-Polio Syndrome," but should be aware that NPP, and possibly even a poliovirus- induced "minor illnesses," can be associated with CNS damage and late-onset muscle weakness and fatigue.
Paralytic Versus "Non-Paralytic" Polio: A Distinction without a Difference?
Many clinicians, even some who treat polio survivors, believe that individuals who had non-paralytic polio (NPP) cannot develop post-polio sequelae (PPS) -- fatigue, muscle weakness, joint and muscle pain, cold intolerance and difficulty sleeping, swallowing and breathing – that occur more than 30 years after the acute poliovirus infection. 1
The common wisdom is that NPP is synonymous with "abortive polio," in which the poliovirus caused a flu-like illness but did not enter the central nervous system (CNS) and therefore neither damaged nor killed neurons. 2 Of course, autopsies were not performed on humans diagnosed with NPP to determine if there were damage to the CNS.
However, there is significant circumstantial evidence that NPP was associated with CNS damage. For example, it was reported in 1953 that 39% of those diagnosed with NPP had measurable weakness on manual muscle testing in at least one muscle group. 3 A 1954 paper, “The Infrequent Incidence of Nonparalytic Poliomyelitis,” documented that 89% of polio survivors who were acutely "persuasively nonparalytic" had "very definite muscle weakness" as long as three years after the diagnosis of NPP. 4
The "Summer Grippe" and CNS Poliovirus Lesions
A less circumstantial case for the neuropathic nature of NPP came in 1947 from Cincinnati, Ohio. 1946 had been America's worst polio epidemic to date, the death rate rising to the century's highest level. But from June through September of 1947, only 40 cases of polio were reported in Cincinnati when there had been 167 cases during a previous summer. Coincidentally, for at least four weeks during August and early September, pediatricians saw large numbers of children with an illness they called "summer sore throat" or "Summer Grippe." 5
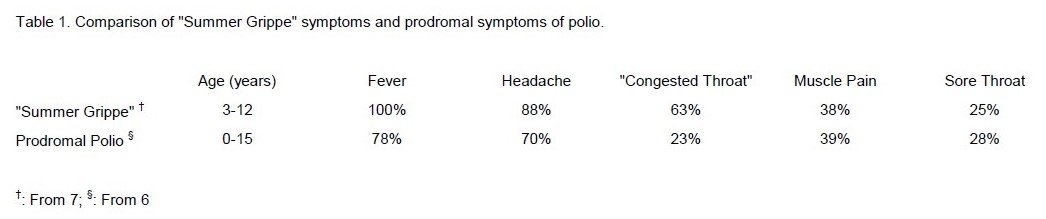
The onset of "Summer Grippe" was sudden, with fever, headache, sore throat, and muscle pain -- the symptoms characteristic of abortive polio and the first phase of a poliovirus infection of the CNS. 6
(Table 1) Children with "Summer Grippe" were between one and ten years old, were not sick enough to be admitted to hospital and saw their physician once if at all. But the epidemic was large.
Albert Sabin, at The Children's Hospital in Cincinnati, reported that there were at least "10,000 cases of this illness" and that "in some parts of the city hardly a child escaped."
Why was Sabin, a preeminent polio researcher, interested in the "Summer Grippe?" Because some of the children had spinal or nuchal (neck) rigidity, the "red flag" symptoms that would trigger hospitalization and a presumptive diagnosis of "poliomyelitis."
However, since "Summer Grippe" symptoms were not associated with paralysis and disappeared within a week, pediatricians were not interested in hospitalizing children. But Sabin was. He was aware that paralytic polio among American troops in the Philippines from 1944 to 1946 was accompanied by thousands of cases of "fever of unknown origin (with) pleocytosis." 7
Sabin also knew of an unusually mild polio outbreak in Denmark in 1934 where, although only 27 patients were paralyzed, 600 more reported having just a "slight fever." 5 He also recalled the unusual increase in influenza in Copenhagen during August and September of 1934 that was said to be "probably due to infection with poliomyelitis virus" since the "flu" was associated with over 100 cases of polio. 5
Since summer outbreaks of influenza are uncommon, Sabin wondered if a mild form of the poliovirus could have caused fever in the Philippines, "flu" in Denmark, and was causing "Summer Grippe" in Cincinnati. 8
Clinical studies. From August 22 to September 9 1947, Sabin admitted 13 children to The Children's Hospital who had had a sudden onset of "Summer Grippe" symptoms. 5 The children had a fever of about 103, almost all were "listless," had headaches, and most had sore throats. (Table 1) The children had lumbar punctures on admission and throat swabbings and stool specimens collected on three consecutive days. Eight children (ages 3 to 12 [x=6] years) were diagnosed as having "Summer Grippe," three of whom had pleocytosis (40 - 150 [x=98] leukocytes/mm3 of CFS). One child had "dysentery," another "rhinitis" and a third "atypical pneumonia." Two children, ages 4 and 6 years, developed "definite nuchal-spinal rigidity for a brief period, which might have been missed if the children had remained at home," had pleocytosis (32 and 474 leukocytes/mm3), and were diagnosed as having non-paralytic polio. None of the patients was seriously ill and they all left the hospital in about nine days.
Sabin found antibodies to the Type II ("Lansing") poliovirus in five "Summer Grippe" patients, in the child with "rhinitis," and in one child with NPP. But antibodies could have been present because the children were exposed to poliovirus during the epidemic of 1946. So Sabin followed the accepted protocol for documenting that a Type II poliovirus had indeed been present in the children: he inoculated 27 rhesus monkeys with the material collected from throat swabbings and stool specimens, observed the animals for weakness or paralysis, and sacrificed them within 35 days.
Specimens from four "Summer Grippe" patients caused infection in monkeys. Of the 18 animals inoculated, one monkey died after developing peritonitis, one was paralyzed and the rest appeared well. When the animals were sacrificed, 50% had spinal cord and/or brain lesions typical of poliovirus infection. Specimens from three patients damaged spinal cord motor neurons while specimens from four caused lesions characteristic of the poliovirus in the midbrain reticular formation, medulla, hypothalamus, thalamus, dorsal vagal motor nuclei, vestibular nuclei, the olives and substantia nigra. 9,10 Specimens from only one of the two children diagnosed with NPP caused paralysis in the inoculated monkeys and damaged neurons in the spinal cord and medulla. 9
However, when Sabin inoculated monkeys with specimens from seven additional patients from Cincinnati diagnosed with NPP during August, 1947, only one monkey became paralyzed and the others had no evidence of CNS damage. 5
Sabin concluded from these findings that a "low-virulence" Type II poliovirus caused the flu-like symptoms of "Summer Grippe." However, although Sabin's low-virulence poliovirus did not cause even muscle weakness in humans, it lesioned the monkey CNS nearly twice as frequently as did the virus causing typical NPP in Cincinnati that same summer. What is more, Sabin's low virulence poliovirus did something even the most virulent paralytic poliovirus did not do: sicken at least 10,000 children. At its worst the “typical” poliovirus in Cincinnati had felled only 167.
Sabin versus Bodian.Yet not everyone agreed with Sabin that a low virulence Type II poliovirus caused the "Summer Grippe," not even David Bodian, editor of The American Journal of Hygiene, which published his paper. Bodian told Sabin in a letter that the evidence supporting his conclusion was "very far from being satisfactory" and that the paper would be "subject to serious criticisms." 9
Bodian, a Johns Hopkins neuropathologist, had performed dozens of postmortems on humans who had contracted polio and scores of autopsies on monkeys intentionally infected with poliovirus. Bodian discovered that damage to specific neurons in the brain stem and anterior horn of the spinal cord -- the same lesions Sabin found as a result of inoculating monkeys with specimens from "Summer Grippe" patients – is the characteristic histopathologic "signature" of poliovirus infection of the CNS. 10
Bodian also documented that 60% of motor neurons had to be killed by the poliovirus for any muscle weakness -- let alone paralysis -- to be clinically apparent: "The evidence from experimental work and from human material is overwhelmingly clear on this point. Neuronal and inflammatory lesions may be regularly found in any susceptible center, including the anterior horn of the spinal cord, in individuals who have never exhibited symptoms." 10 Bodian should have been the one scientist to readily accept Sabin's claim that a poliovirus could cause "Summer Grippe" symptoms and kill neurons, although not enough neurons to cause paralysis, weakness or even nuchal-spinal rigidity. Bodian did not. Bodian wrote to Sabin that a "causal relationship" between the poliovirus and "Summer Grippe" had not been proved saying, "it is equally plausible to assume that the (polio) virus was found in accidental relationship with the illness." 9
Sabin wrote back that specimens collected "during the same period" from 24 additional patients with NPP did not cause CNS lesions in monkeys, supporting his claim that there was a different poliovirus causing "Summer Grippe," and that damaged neurons in monkeys more frequently than did the NPP poliovirus. 9
But Sabin could not actually prove a causal relationship between a his "low virulence" poliovirus and the "Summer Grippe," admitting to Bodian, "It is, in fact...a matter of probable guilt by association." 9 As Sabin became occupied with development of the oral polio vaccine there is no evidence, from his publications or private papers, that he continued to argue "that in Cincinnati in 1947 there were in circulation at the same time strains of high and low virulence" poliovirus, the latter causing the "Summer Grippe." However, Sabin's own research would eventually support this hypothesis.
In 1949 Bodian discovered that not just the Type II poliovirus, but three distinct poliovirus immunotypes, caused illness in humans. 11 In 1952 Sabin tested specimens saved since 1947 from his paralytic polio patients (but, unfortunately, not from the "Summer Grippe" children) for the newly identified poliovirus immunotypes. 12 Sabin discovered that all of the 1947 specimens from the paralytic polio patients were positive for Type I ("Brunhilde") poliovirus antibodies, thus documenting that Type I poliovirus -- not Type II -- was responsible for paralytic polio in Cincinnati in 1947.
Still, it was not proved that a "low virulence" Type II poliovirus caused the "Summer Grippe." However, an observation with which Sabin began his paper describing the "Summer Grippe" would point the way to a causal connection: when the epidemic of "Summer Grippe" was "at its peak by the end of August, there were not more than 40 reported cases of poliomyelitis." But, when the "Summer Grippe" was largely over by the middle of September, the number of polio cases increased, reaching a total of 170; 77% of polio cases in Cincinnati during 1947 occurred only when the "Summer Grippe" epidemic was over. It appeared that Sabin's putative low virulence, Type II "Summer Grippe" poliovirus somehow prevented the children of Cincinnati from being infected with a high virulence Type I strain that caused the additional 130 cases of "typical" polio, but only when "Summer Grippe" virus was gone. "Interference" between different poliovirus immunotypes would ultimately support a low virulence Type II poliovirus as the cause of the "Summer Grippe" in 1947.
Iceland Disease: Another Form of "Non-Paralytic" Polio?
In September of the next year, three cases of paralytic polio were diagnosed in Akureyri, Iceland. Although not another case of polio was documented, more than 1100 additional Icelanders would report symptoms typical of paralytic polio infection (fever, neck pain, muscle weakness and a few cases of paralysis) as well as symptoms not usually associated with polio (e.g., tingling and numbness) 13 Specimens from four Icelanders were sent to Bodian's laboratory for testing, but poliovirus antibodies were not found. Yet, physicians in Iceland concluded that there were only two possible causes for what has come to be known as Iceland Disease (ID): "Either a strain of (poliovirus) of low virulence was responsible for this epidemic" or "some unknown neurotropic virus has been present." 13
Evidence supporting a low virulence poliovirus as the cause of ID did not come for six more years. In 1955 there was a large polio epidemic in Iceland caused by Type I poliovirus along with new outbreaks of ID. 14 Remarkably, no cases of polio were reported in ID-affected towns in spite of only 7% of children having antibodies to Type I poliovirus, as compared to 86% of the children in the polio epidemic areas. However, 100% of the children in towns with ID had antibodies to Type II poliovirus. As Sabin thought occurred in Cincinnati, children in Iceland apparently had been exposed to a low virulence Type II poliovirus that damaged the CNS and caused symptoms of Iceland Disease, but prevented infection by a high virulence Type I poliovirus.
How could infection by one poliovirus strain prevent infection by another without protective antibodies? That answer came during the 1959 Singapore trial of Sabin's oral polio vaccine. 15 Children were vaccinated with Sabin's three types of live, attenuated poliovirus. The Type II poliovirus was always found to be "dominant" over the others, the only type that always entered the blood. It was concluded that the Type II poliovirus actually "interfered" with other polioviruses (even the naturally-occurring Type I poliovirus that was causing the 1959 Singapore polio epidemic) stopping Types I and III from entering the blood and thus preventing CNS infection even in those without poliovirus antibodies.
Such interference of Type II poliovirus with both Types I and III has been documented repeatedly since 1959. 16 Therefore, it appears that children in Iceland in 1955 did not develop paralytic polio because a low-virulence Type II poliovirus interfered with infection by a high-virulence Type I poliovirus, but at the price of contracting another neuropathic illness, Iceland Disease. In 1947, a low- virulence Type II poliovirus apparently protected the children of Cincinnati from paralysis by preventing a high-virulence Type I poliovirus from entering the blood, but at the price of the "Summer Grippe."
"Non-Paralytic" Polio: Revisiting the Common Wisdom
New muscle weakness and non-paralytic polio. Sabin's finding that there is a "mild" Type II poliovirus that damaged neurons in the spinal cord and brain without causing nuchal rigidity or paralysis, and evidence that a Type II poliovirus of "low virulence” was responsible" for the ID outbreaks, are important as we try to understand the pathophysiology of post-polio syndrome (PPS) today.
As early as 1941, Bodian cautioned that "the clinical diagnosis of a non-paralytic case (of polio) may rest on the failure to detect minimal degrees of muscle weakness," predicting the 1953 finding that at least 39% of those diagnosed with NPP in fact had demonstrable muscle weakness. 3,17 Bodian showed that paralytic and non-paralytic polio are not separate entities but a single process whose pathophysiology and clinical manifestations lie on a continuum. He reported that the appearance of paralysis and its severity increased as the percentage of motor neurons killed increased; rhesus monkeys could have apparently normal muscle function with only 40% of their anterior horn cells remaining. 18
In 1997 McComas supported this conclusion, finding the “limbs not considered to have been involved" in paralytic polio survivors had lost on average 40% of their motor units. 19 These findings contradict the common wisdom that the absence of paralysis is synonymous with the absence of CNS damage, and the notion that NPP survivors' motor neurons could not be experiencing metabolic failure and death to which late-onset muscle weakness has been attributed. 19
New fatigue and non-paralytic polio. The focus of PPS research during the past 15 years on new muscle weakness has ignored the most frequently reported and most disabling late-onset symptom, fatigue. 1
Certainly "brain fatigue," with its associated cognitive symptoms, can not be attributed to spinal motor neuron damage. 20 Bodian made scores of post-mortem comparisons between poliovirus-induced spinal cord and brain lesions. In animals without paralysis, he found that "lesions in the brain are more extensive and numerous than in some monkeys which did develop paralysis" and that some "animals with non-paralytic poliomyelitis do not have any lesions in the spinal cord but have a characteristic distribution of lesions in the brain ." 21, 22 (Notably, the brain lesions Bodian documented were in the same areas damaged in monkeys inoculated with specimens from Sabin's "Summer Grippe” patients.)
Bodian stated that "although non-paralytic infection may be associated with severe neuronal damage in the spinal cord," the poliovirus "is capable of producing an encephalitis, with or without associated clinical symptoms, in the absence of any pathological change in the spinal cord" (italics Bodian's). 17 Bodian also found that the "degree of involvement of brain centers" in humans having paralytic polio is comparable to the brain lesions in animals with non-paralytic polio 18.
Bodian concluded that "all available evidence shows conclusively that every case of poliomyelitis, human or experimental, exhibits lesions of the brain,” and that, "As far as the pathologist is concerned all cases of poliomyelitis are 'encephalitic'." 22
The finding that lesions in the reticular activating system can be the only damage resulting from poliovirus infection may explain:
1) Why fatigue is reported more frequently than muscle weakness in both paralytic and non- paralytic polio survivors,
2) The lack of correlation between the severity of late-onset fatigue and the number of limbs paralyzed, hospital admission or length of hospital stay, impaired breathing or use or respirator during the acute polio episode, and
3) The neuropsychologic, neuroendocrine, neuroanatomic, and EEG abnormalities associated with post-polio fatigue and the occurrence of central sleep apnea and abnormalities of sleep architecture in polio survivors. 1,20,23-28 With the occurrence of late-onset fatigue, we are seeing today what Bodian predicted in 1949: that symptoms resulting from "lesions in the central gray of the mid-brain and hind-brain and in the mid-brain tegmentum and reticular formation" would be more often observed if they were not overshadowed by "paralysis of skeletal muscles." 10
Prevalence of poliovirus-induced illness. Unfortunately, it is impossible to know how many Americans had their CNS damaged by the poliovirus, damage that set the stage for PPS. Although infectious disease texts state there is a 1:10:50 ratio of paralytic polio, non-paralytic polio, and a "minor illness" with symptoms identical to the "Summer Grippe,” the data do not support this claim. 2,29 In Cincinnati during 1947, the ratio of paralytic to non-paralytic cases was 1:1.2 while the ratio of paralytic polio patients to "Summer Grippe” cases was at least 1:106 5, 7 Across the U.S., the incidence of reported NPP cases varied widely depending on location and year, ranging from 0% to 80% of cases during the 1940’s (mean ratio of paralytic to non-paralytic cases: 7:3) to 25% - 65% during the 1950’s (mean ratio of paralytic to non-paralytic cases: 1:1). 4, 7 (R. Bruce, C.D.C., personal communication.)
There are a several of factors that caused the marked variability in reported cases of paralytic and non-paralytic polio:
• Patients did not present for diagnosis. Failure to present for diagnosis may have resulted from symptoms being very mild or not seen as indicative of poliovirus infection (especially in infants) and patients, especially those who were poor, not having access to or having to travel for medical care. A study of polio diagnoses in a San Francisco city hospital from 1950 to 1953, and the reported cases of polio in Cincinnati during 1947, show that 15% more paralytic and 121% more non-paralytic polio cases were diagnosed among patients living in the city versus those living in outlying areas. 4, 7
Urban patients were thought to be more likely to present for diagnosis, being nearer to hospitals, versus those living in areas where they had to travel for medical care. Those with mild symptoms -- as in the "Summer Grippe,” non-paralytic polio or even mild paralytic polio, especially in infants and young children -- may never have been diagnosed. Although diagnosis may have been more accurate and reporting to public health authorities more likely at city hospitals, and especially at university medical centers such as Sabin's in Cincinnati, the number of cases of both paralytic and non-paralytic polio reported by rural hospitals would have been underestimates of the true incidence:
• Paralytic polio was underdiagnosed. Studies document the underdiagnosis of paralytic polio. 3,4 Shaw and Levin reported that "mild degrees of muscle weakness may be easily overlooked” if manual muscle testing were employed without a functional assessment of strength: “Many patients who are eager for activity, who can readily walk out of the hospital, and who are persuasively non-paralytic will thus be found to have very definite muscle weakness which cannot be detected while they are recumbent in bed." 4 The failure to detect muscle weakness was also attributed to "too short a period of observation without opportunity for follow-up;" eighteen months to 6 years after having been diagnosed with non-paralytic polio, 39% of patients were found to have weakness in at least one muscle group; 3,4
• Polio cases were not reported. The number and severity of polio cases during the epidemics may also have prevented overwhelmed physicians from reporting the occurrence of polio, especially NPP, to local public heath authorities. This occurred even at Sabin’s Children’s Hospital in Cincinnati. (Dr. J. Englert, personal communication.) Even if polio cases were reported locally, between 1915 to 1934 a varying number of states failed to report cases to the federal government, thereby preventing documentation of the true incidence of polio and even the extent of large polio outbreaks, such as the1916 epidemic. 7 What is more, the C.D.C. did not separately tabulate non-paralytic and paralytic polio cases until 1951, and did not itself require the reporting of polio cases until the late 1950’s. (R. Bruce, C.D.C., personal communication.)
These factors have not only caused inaccuracies in archival polio incidence and prevalence data, but also they have impaired the ability of recent surveys to accurately estimate the actual number of living polio survivors. For example, the 1987 National Health Interview Survey (N.H.I.S.) relied on respondents to report whether they had had polio and if they had paralytic or NPP. 30 The N.H.I.S. estimated that there are 641,416 living American paralytic polio survivors, 832,852 NPP survivors and 159,919 who reported they had had polio but did not know whether that had been paralyzed. This survey documented 1 paralytic case for 1.3 non-paralytic cases, a ratio similar to that reported during the 1950's but much lower than the 7:3 ratio of paralytic to non-paralytic cases reported during the 1940's.
The fact that 10% of the N.H.I.S. respondents did not know whether they had been paralyzed makes clear that retrospective surveys, which rely on patient reports of early-life symptoms and diagnoses, may not be able to accurately determine the prevalence of paralytic polio, let alone the prevalence of NPP or a "minor illness" such as the "Summer Grippe.”
“Polio suspects” and PPS. It may be possible to control for the sources of diagnostic and reporting error described above and approximately estimate the number of living Americans who did have a "minor illness” and thereby estimate the total number of polio survivors who had CNS damage and therefore are at risk for PPS today. As early as 1935, the Mayo Clinic’s centralized records-linkage system recorded not only cases of paralytic and non-paralytic polio, but also what were called polio "suspects . . ."
". . . a term used by the physicians in polio epidemic years to describe persons with an acute fibrile illness suspiciously similar to polio but without paresis or evidence of central nervous system involvement from clinical history and/or cerebrospinal fluid examination. Many of these persons were family members and contacts of cases. Some may have had abortive polio and may constitute the 'tip of the iceberg,' since so many cases of polio never came to the attention of the physician." 31
Although the population-based Mayo data are not representative of polio incidence in other regions of the country, especially in urban centers, they do significantly reduce, if not eliminate, errors resulting from improper diagnosis and underreporting. Between 1935 and 1955, for each paralytic polio patient, 0.65 polio "suspects" were recorded in the Mayo system. Applying this ratio to the N.H.I.S. estimate of 641,416 paralytic polio survivors, there would be at least 416,920 living polio "suspects" having had symptoms similar to those of the "Summer Grippe." Since 38% of Sabin’s "Summer Grippe" patients had pleocytosis, indicating CNS involvement, 158,430 Americans would be at risk for PPS in addition to the estimated 1.63 million paralytic and non-paralytic polio survivors.
Clinical Implications
“Non-paralytic” polio and “Post-Polio Syndrome." The diagnostic criteria for "Post-Polio Syndrome" reflect the principal clinical concerns of the 1950’s and the 1980’s: acute paralysis and new muscle weakness, respectively. Post-Polio Syndrome criteria require the "onset of new weakness" in an individual with a "prior episode of paralytic polio confirmed by history, physical exam," as well as EMG changes "consistent with prior anterior horn cell disease." 32 Unfortunately, these criteria do not take into account the studies described above which make clear that non-paralytic polio -- and even a "minor illness" such as the "Summer Grippe” -- can be associated with the death of neurons in the spinal cord and brain that sets the stage for late-onset symptoms.
Recent studies make clear that NPP survivors do have late-onset symptoms. A population-based study of 828 polio survivors found that new muscle weakness and fatigue were reported, respectively, in 38% and 34% of those who had been paralyzed, and in 14% and 21% of those who had had NPP. 23 A study of 34 sets of twins found PPS symptoms in 71% of the twins who had had paralytic polio and "PPS-like symptoms" in 42% who had had no symptoms of paralysis. 33 The presence of PPS- like symptoms, as well as muscle biopsies "showing long-standing signs of denervation" in some of the non-paralyzed twins, caused the authors to conclude that the acutely non-paralyzed twin “had also suffered subclinical non-paralytic polio (and that) symptoms of PPS might also be sequelae of non-paralytic polio." Indeed, 13% of the non-paralyzed twins had been acutely diagnosed as having “non-paralytic polio.” 34 Further, the 1987 N.H.I.S. found that 28% of NPP survivors reported new health problems related to polio. 30 45% more paralytic polio survivors reported limitations as compared to those who had had NPP, while NPP polio survivors reported 46% more limitation in life activities than did the general population. (Table 2)
EMG changes indicative of denervation may not be present in those who had "Summer Grippe,” NPP or even in those who had acute transient paralysis. Sabin stated that "transitory" and reversible paralysis may be seen because "not all nerve cells attacked by the poliomyelitis virus are irreversibly damaged." 35 Bodian documented that motor neurons did recover function after poliovirus infection even if all of the intracellular organelles, except for the nucleus, had been destroyed. 10 Thus, an individual could have been acutely paralyzed without neuronal death or axonal degeneration, the poliovirus-infected neurons recovering and restoring muscle function without EMG evidence of chronic denervation. Such a scenario would be more likely in those who had been weakened or mildly paralyzed acutely and especially in those who had NPP or "Summer Grippe."
However, it is important to note that neurons which recovered from poliovirus infection were internally damaged, their cellular apparatus for oxidative metabolism, protein synthesis, neurotransmitter packaging and axonal transport likely having been significantly impaired. 36,37 Bodian suggested that such poliovirus-damaged neurons “may be vulnerable for life to metabolic factors such as changes of senescence;” this vulnerability and eventual metabolic failure are thought to be the likely causes of late-onset muscle weakness and fatigue in polio survivors with or without acute evidence of paralysis or EMG evidence of denervation. 19,26,38-40.
Therefore, "Post-Polio Syndrome" should not be used as the generic descriptor for late-onset problems in polio survivors, since its diagnostic criteria exclude those without a history of paralysis, EMG evidence of denervation and new muscle weakness. The Post-Polio Syndrome definition should either be modified to include other frequently reported and disabling symptoms, especially fatigue, or its name should be changed to "Post-Polio Muscle Weakness Syndrome." In either case, the requirement of a history of paralytic polio and EMG evidence of denervation should be removed.
Clinicians need to explain to their patients that NPP, and possibly even a poliovirus-induced "minor illness" such as “Summer Grippe,” can be associated with CNS damage and late-onset symptoms. Non-paralytic polio survivors with PPS symptoms should apply the conservative techniques found to be successful in managing paralytic polio survivors’ PPS: reducing physical and emotional stress, using appropriate assistive devices, conserving energy, adequate rest and the pacing of activities. 41- 4
References
Bruno RL, Frick NM. Stress and "Type A" behavior as precipitants of Post-Polio Sequelae. In LS Halstead and DO Wiechers (Eds.):Research and Clinical Aspects of the Late Effects of Poliomyelitis. White Plains: March of Dimes Research Foundation, 1987.
Hoepich PD, Jordan MC. Infectious Diseases. Philadelphia:Lippincott, 1989.
Moskowitz E, Kaplan LI. Follow-up study in seventy-five cases of nonparalytic poliomyelitis. JAMA 1953; 52:1505-1506.
Shaw EB, Levin M. The Infrequent Incidence of Nonparalytic Poliomyelitis. J Pediatrics 1954; 44:237-243.
Sabin AB, Steigman AJ. Poliomyelitis virus of low virulence in patients with epidemic "Summer Grippe or Sore Throat." Am. J Hyg 1949; 49:176-193
Weinstein L, Shelokov A, Seltzer R, Winchell GD. A comparison of the clinical features of poliomyelitis in Adults and children. N.E.J.M 1952; 246:296-302.
Sabin AB. Epidemiologic patterns of poliomyelitis in different parts of the world. In Poliomyelitis. Lippincott:Philadelphia, 1949.
C.D.C. Update. Outbreak of Influenza A Infection -- Alaska and the Yukon Territory, July-August 1998. M.M.W.R. 1998; 47:685-688.
Papers from the The Hauck Center for the Albert B. Sabin Archives, Cincinnati Medical Heritage Center, University of Cincinnati Medical Center.
Bodian D. Histopathological basis of clinical findings in poliomyelitis. Am J Med 1949; 6:563-578.
The committee on typing of the National Foundation for Infantile Paralysis. Immunologic classification of poliomyelitis viruses. Am. J Hyg 1951; 54:191-204.
Sabin AB. Transitory appearance of Type 2 neutralizing antibody in patients infected with Type 1 Poliomyelitis virus. J Exp Med 1952; 96:99-106.
Sigurdsson B, Sigurjonsson J, Sigurdsson HJ, et al. A disease epidemic in Iceland simulating poliomyelitis. Am J Hyg 1950; 52:222-238.
Sigurdsson B, Gudnadottir M, Petursson G. Response to poliomyelitis vaccination. Lancet 1958; i:370-371.
Hale JH, Lee LH, Gardner PS. Interference patterns encountered when using attenuated poliovirus vaccines Brit.Med J 1961; 2:278-732.
Patriarca PA, Wright PR, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in Developing countries: Review. Rev Infect Dis 1991; 13:926-939.
Bodian D, Howe HA. The pathology of early arrested and non-paralytic poliomyelitis. Bull Johns Hopkins Hosp 1941; 69:135-147.
Bodian D. Poliomyelitis: Pathologic anatomy. In Poliomyelitis. Lippincott: Philadelphia, 1949.
McComas AJ, Quartly C, Griggs RC. Early and late losses of motor units after poliomyelitis. Brain 1997; 120:1415-1412.
Bruno RL, Creange SJ, Frick NM. Parallels between post-polio fatigue and chronic fatigue syndrome: A Common pathophysiology? Am J Med 1998, 105 (3A):66-73.
Bodian D, Howe HA. Experimental nonparalytic poliomyelitis: Frequency and range of pathological involvement. Bull Johns Hopkins Hosp 1945; 76:1-17.
Bodian D. Poliomyelitis: Neuropathologic observations in relation to motor symptoms. JAMA 1947 134, 1148-1154.
Ramlow J, Alexander M, LaPorte R, et al. Epidemiology of the Post-Polio Syndrome. Am J Epidemiology 1992, 136:769-786.
Bruno RL, Creange SJ, Zimmerman JR, Frick NM. Elevated plasma prolactin and EEG slow wave power in Postpolio fatigue: Implications for a dopamine deficiency underlying chronic fatigue syndromes. J CFS 1998; 4:61-76.
Bruno RL. Chronic fatigue, fainting and autonomic dysfunction: Further similarities between post-polio Fatigue and Chronic Fatigue Syndrome? J CFS 1997; 3:107-117.
Bruno RL, Frick NM, Creange SJ, Molzen T, Lewis T, Zimmerman JR. Polioencephalitis and the brain Fatigue generator model of post-viral fatigue syndromes. J CFS 1996; 2:5-27.
Siegel H, McCutchen C, Dalakas MC, et al. Physiologic events initiating REM sleep in patients with the Postpolio syndrome. Neurol 1999; 52:516-522.
Dean AC, Graham BA, Dalakas M, Sato S. Sleep apnea in patients with postpolio syndrome. Ann Neurol 1998; 43:661-664.
Young NA. Poliovirus In GL Mandell, RG Douglas, JE Bennett (Eds.): Principles and Practice of Infectious Diseases. New York: John Wiley and Sons, 1979.
Parsons PE. National Health Interview Survey. Hyattsville, Maryland: National Center for Health Statistics, 1989.
Codd MB, Mulder DW, Kurland LT, et al. Poliomyelitis in Rochester, Minnesota, 1935-1955: Epidemiology And long-term sequelae. In LS Halstead and DO Wiechers (Eds.): Late Effects of Poliomyelitis. Miami: Symposia Foundation, 1985.
Halstead LS. Assessment and differential diagnosis for post-Polio Syndrome. Orthopedics 1991; 14:1209- 1217.
Nee L, Dambrosia J, Bern R, et al. Post-Polio Syndrome in twins and their siblings: Evidence that Post- Polio Syndrome can develop in patients with non-paralytic polio. Ann NY Acad Sci 1995:378-380.
Herndon CN, Jennings RG. A twin family study of susceptibility to poliomyelitis. Am J Human Genetics 1951; 3:17-46.
Sabin AB, Ward R. Nature of non-paralytic and transitory paralytic poliomyelitis in rhesus monkeys Inoculated with human virus. J Exp Med 1941; 73:757-770.
Bodian D, Horstmann DM. Polioviruses. In Horsfall D (Ed.): Viral and Rickettsial Infections of Man. Philadelphia: Lippincott, 1965.
Pezeshkpour GH, Dalakas MC. Long-term changes in the spinal cords of patients with old poliomyelitis. Arch Neurol 1988; 45:505-508.
Bodian D. Motorneuron disease and recovery in experimental poliomyelitis. In LS Halstead, DO Wiechers (Eds.): Late Effects of Poliomyelitis. Miami, Symposia Foundation, 1985.
Weichers DO. Acute and latent effect of poliomyelitis on the motor unit as revealed by electromyography. Orthopedics 1985; 8:872-872.
Bruno RL, Frick NM, Cohen, J. Polioencephalitis, stress and the etiology of Post-Polio Sequelae. Orthopedics 1991; 14 (11):1269-1276.
Young GR. Energy conservation, occupational therapy and the treatment of post-polio sequelae. Orthopedics 1991; 14:1233-1239.
Agree JC, Rodriguez AA. Neuromuscular function in polio survivors. Orthopedics 1991;14:1343-47
Bruno RL, Frick NM. The psychology of polio as prelude to Post-Polio Sequelae: Behavior modification and psychotherapy. Orthopedics 1991; 14:1185-1193.
Creange SJ, Bruno RL. Compliance with treatment for Post-Polio Sequelae: Effect of Type A behavior, Selfconcept and loneliness. Am J PM&R 1997; 76:378-382.
Peach PE, Olejnik S. Effect of treatment and noncompliance on Post-Polio Sequelae. Orthopedics 1991; 14:1199-1203.